It comprises sodium cation and hypochlorite anion. Sodium hypochlorite is first produced in 1789 by Claude Louis Berthollet in his laboratory in Paris France.
 Sodium Hypochlorite Solution Naocl Cas No 7681 52 9 9 10 5 L Can At Rs 100 Litre Jlpl Industrial Area Mohali Id 18479342762
Sodium Hypochlorite Solution Naocl Cas No 7681 52 9 9 10 5 L Can At Rs 100 Litre Jlpl Industrial Area Mohali Id 18479342762
Sodium hypochlorite commonly referred to as.

What is sodium hypochlorite. 58 Monday March 26 2012 Rules and Regulations 11192013 EN English 57 Sodium Hypochlorite. Sodium hypochlorite NaOCl is a solution made from reacting chlorine with a sodium hydroxide solution. Sodium Hypochlorite 5 wv Safety Data Sheet according to Federal Register Vol.
What is Sodium Hypochlorite. Thyristor controlled rectifier units provide LV variable voltage DC supplies to the cells thereby controlling the rate of production of sodium hypochlorite by electrolysis within the cells. Sodium Hypochlorite is a chlorine compound often used as a disinfectant or a bleaching agent.
How to Use Sodium Hypochlorite Bleach the Right Way. Household bleach solutions contain 5 sodium hypochlorite liquid bleach. Na OCl- NaOCl sodium hypo- sodium cation chlorite hypochlorite anion The most common method for producing sodium hypochlorite is to react chlorine with sodium hydroxide NaOH.
Sodium hypochlorite is an inorganic compound with the chemical formula NaOCl. The sodium hypochlorite solution is highly toxic undiluted. Sodium hypochlorite NaOCl is a compound that can be effectively used for water purification.
It is diluted with water or other filler ingredients to a concentration of about 60 sodium hypochlorite NAClO. It appears as a greenish-yellow solid. If you have an allergy to sodium hypochlorite solution or.
A by-product of the process is hydrogen which is of inadequate purity for. Bleaching powder is chemically Calcium hypochlorite CaClO2. Sodium hypochlorite is an inorganic compound having the chemical formula NaOCl.
Sodium Hypochlorite is the main ingredient in laundry bleach. Known hypochlorites is sodium hypochlorite the active ingredient in bleach. It is known by various other names such as antiformin bleach etc.
The molar mass is 7445 gmol. It is used to clean wounds. It is also known as liquid bleach.
What do I need to tell my doctor BEFORE I take Sodium Hypochlorite. It is used to treat skin ulcers. When was sodium hypochlorite discovered.
It is a greenish-yellow solid at room temperature. Has a pH of 13 and requires that small amounts of acid be added to the pool to neutralize the high pH. This alkaline cleaning agent is commonly referred to as bleach paid link.
This shelf life is not adequate for use in the SWS which requires that the hypochlorite remain at a high enough concentration to. Sodium hypochlorite is an ionic chemical compound with the formula NaOCl. Calcium hypochlorite is relatively stable and has greater available chlorine than sodium hypochlorite.
It is used extensively as a bleaching agent in the textile detergents and paper and pulp industries. The melting point and boiling points of the most common hydrate of this compound pentahydrate form are 18 C and 101 C respectively. The molecular formula for sodium hypochlorite is NaOCl.
Small amounts of diluted chlorine bleach are safe for plants and in some cases even helpful. It is considered to be the sodium salt of hypochlorous acid and is used as liquid bleach an oxidizing agent and a disinfectant when mixed with water. Sodium hypochlorite is a chemical compound with chemical formula NaOCl.
Sodium hypochlorite solution is commonly known as whitening agent or Clorox. It is generally found in its pentahydrate state. What is Sodium Hypochlorite.
It is used on a large scale for surface purification bleaching odor removal and water disinfection. These two reactants are the major co-products from most chlor-alkali cells. Usually provides 10 to 12 available chlorine.
It is used to avoid or treat skin infections. It consists of hypochlorite anion and sodium cation. Sodium hypochlorite is a chemical compound with the chemical formula NaClO.
Especially to plantsIt is the sodium in the bleach that poses the most risk to plants because it interferes with their mineral absorption. It usually appears as a pale greenish yellow dilute solution. These two ions link.
Sodium hypochlorite in 05 wv solution is called Dakins solution and is used as an antiseptic to clean infected topical wounds. In the petrochemical industry sodium hypochlorite is. Sodium hypochlorite is a chemical substance with the formula NaOCl.
Sodium hypochlorite has a long history. Sodium hypochlorite is produced for the treatment of cooling water CW. Sodium hypochlorite is highly reactive and volatile.
Sea water is pumped through strainers to banks of electrochiorination cells. The molecule contains a sodium cation and a hypochlorite anion. At normal pH 6-8 sodium hypochlorite can degrade substantially within 2-3 weeks.
This chemical compound is also used commonly as disinfectant as well. It is also used as an oxidizing agent for organic products. Uses of Sodium Hypochlorite.
Sodium hypochlorite is a staple cleaning agent and the active ingredient in many household cleaners. It has a sweet odour. Sodium Hypochlorite is a chemical compound with the formula NaOClSodium hypochlorite solution commonly known as bleach is frequently used as a disinfectant and as a bleaching agent.


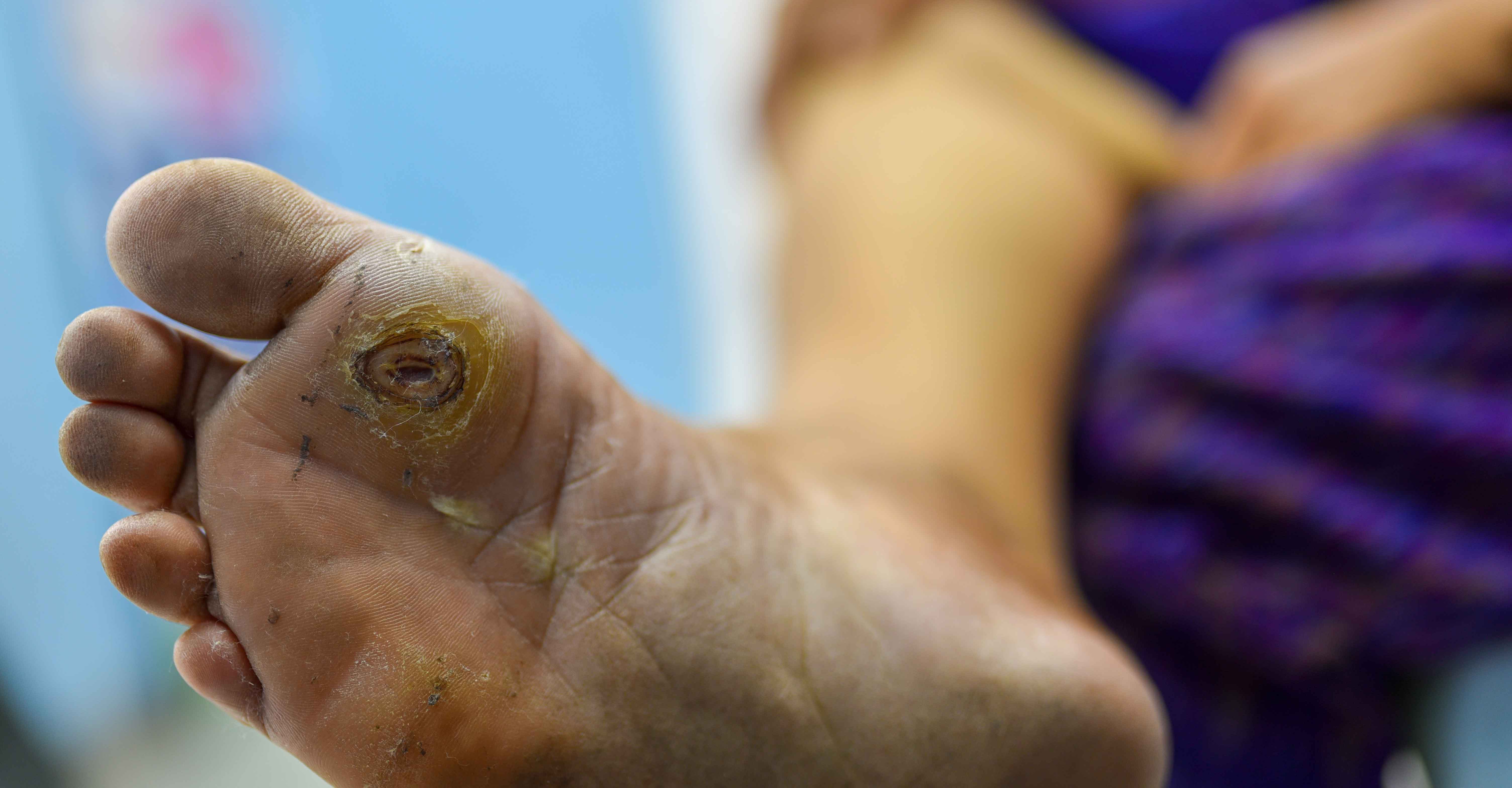









/stages-of-a-cold-sore-outbreak-4173005-5c1a8ad0c9e77c0001e31b0e.png)