The Drug Quality and Security Act HR. Public health requires new.
 Pdf The Drug Quality And Security Act
Pdf The Drug Quality And Security Act
The Compounding Quality Act creates a voluntary compliance regime where compounding pharmacies that voluntarily register as outsourcing facilities will be subject to oversight by the FDA in much of the same way that traditional.
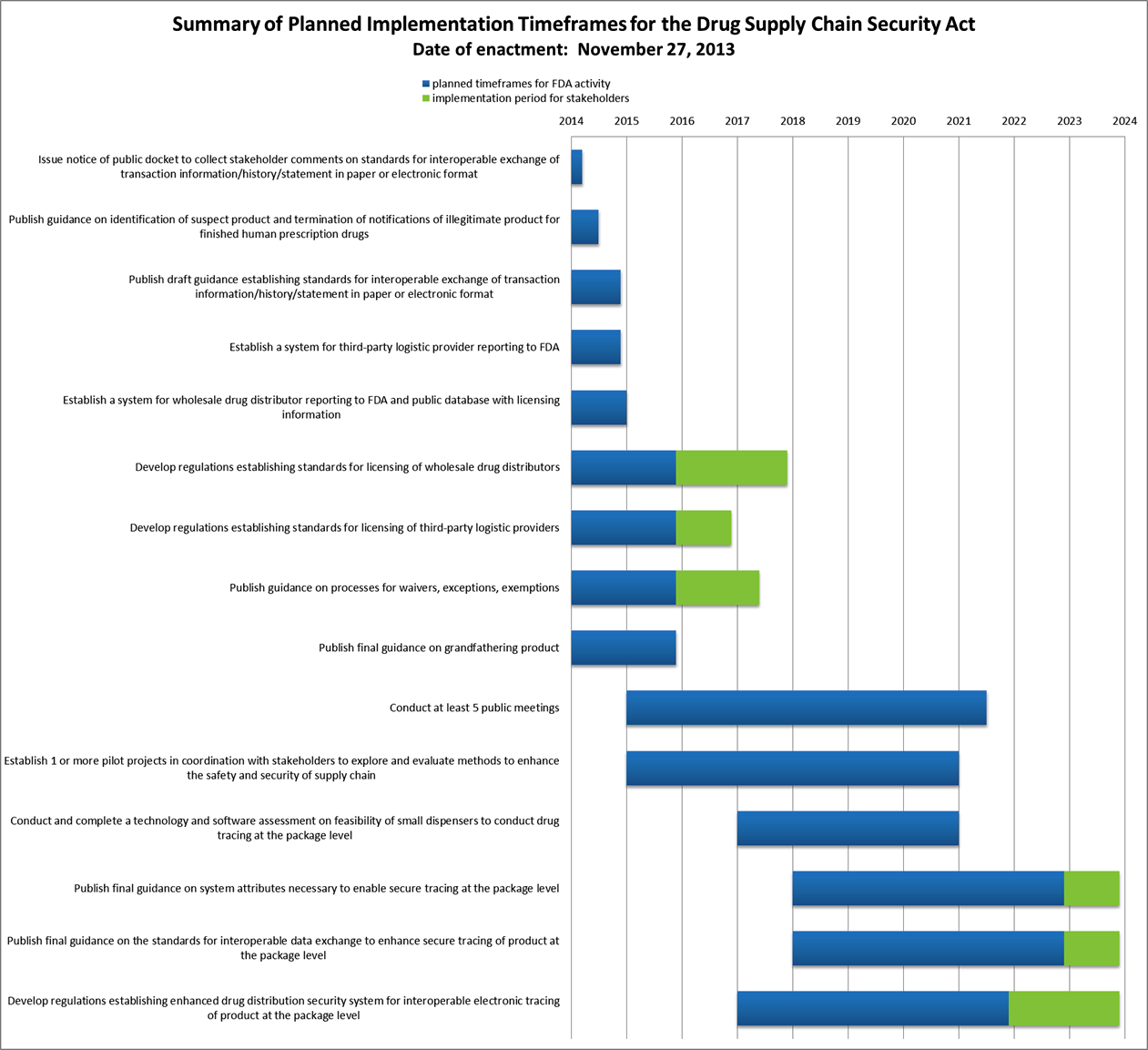
Drug quality and security act. Title II of DQSA the Drug Supply Chain Security Act DSCSA outlines steps to build an electronic. The Drug Quality and Security Act aims to regulate compounding pharmacies and establish a track-and-trace pedigree system for drugs but it falls short on both counts. As passed by the House of Representatives on September 28 2013 and the Senate on November 18 2013.
Shown HerePublic Law No. The Drug Quality and Security Act mind the gaps. 113TH CONGRESS 1ST SESSION S.
The Drug Quality and Security Act DQSA was enacted by Congress on November 27 2013. The Drug Quality and Security Act has two distinct and independent acts. Drug Quality and Security Act 1.
N Engl J Med. Error-proofing in the production process of pharmaceuticals isnt just a matter of good business it has life-and-death implications for consumers. Drug Quality and Securi.
Government Publishing Office Page 127 STAT. The Drug Supply Chain Security Act DSCSA is one of two titles that comprise the Drug Quality and Security Act DQSA which was signed into law by President Obama on November 27 2013. Exempts compounded drugs from new drug requirements labeling requirements and track and trace requirements if the drug is compounded by or under the direct supervision of a.
The Drug Quality and Security Act has two distinct and independent acts. Following this tragedy the Drug Quality and Security Act was enacted a bill that would modify the Federal Food Drug and Cosmetic Act and give more authority to regulate and monitor the manufacturing of compounded medication. 3204 Section 503B of The Food Drug Cosmetic Act.
113-54 11272013 113th Congress Public Law 54 From the US. Title I covers drug compounding and Title II relates to drug supply chain security4 There are 2 major compounding-related changes to current law within Title I of the Drug Quality and Security Act. 1 The DQSA purpose is to address issues related to drug compounding oversight and incorporates a national prescription drug track and trace system inclusive of standards for prescription drug wholesale.
Drug Quality and Security Act was a proposal now a piece of legislation introduced on 2013-09-27 in the House of Commons and Senate respectively of the 113 United States Congress by Fred Upton in relation with. New England Compounding Center meningitis outbreak - Wikipedia. Drug Quality and Security Act consists of 2 Titles.
An outsourcing facility is defined as a facility at one geographic location that Compounds sterile drugs. 3204 a bill to grant the FDA more authority to regulate and monitor the manufacturing of compounding drugs was passed by the Senate on November 27 2013. Drug Quality and Security Act Drug Quality and Security Act Act Details.
The Drug Quality and Security Act HR. Administrative law and regulatory procedures Administrative remedies Advisory bodies Business records. One change involves Section 503A of the Food Drug and Cosmetics Act FDCA24 Sec-.
1 the Compounding Quality Act and 2 the Drug Supply Chain Security Act. The Compounding Quality Act creates a voluntary. Drug Quality and Security Act overviews the new mandate and its implications including implementation strategies for track-and-trace programs along with presenting a fuller understanding of the mechanics of intergovernmental policies and oversights.
To that end the 2013 Drug Quality and Security Act in large part requires new mandates on tracking and tracing chain of custody in the supply chain. 1 the Compounding Quality Act and 2 the Drug Supply Chain Security Act. Drug Compounding - Compounding Quality Act - Sec.
Drug Quality and Security Act - Title I. Ll To amend the Federal Food Drug and Cosmetic Act with respect to human drug compounding and drug supply chain security and for other purposes. After the date of enactment of the Drug Supply Chain Security Act establish by regulation standards for the licensing of persons under section 503e1 as amended by the Drug Supply Chain.
102 Amends the Federal Food Drug and Cosmetic Act FFDCA with respect to the regulation of compounding drugs. 587 Public Law 113-54 113th Congress An Act To. Drug Quality and Security Act The Drug Quality and Security Act adds additional requirements that are causing pharmaceutical companies to adjust their.
In November 2013 President Barack Obama signed the Drug Quality and Security Act aimed at regulating compounding pharmacies and establishing a track-and-trace pedigree system for drugs.
